
Previous Projects
2008-09
2007-08
2006-07
2005-06
2004-05
2003-04
Archived Projects |
Undergraduate
Research
Projects 2009-10 |
Opportunities
exist for undergraduate engineering students to work on independent
research projects with Biomedical Engineering faculty. The opportunities
vary dependant on funding and projects that are available. Some opportunities
are listed below. Please contact the individual faculty member for more details about the project. If you don't see a project
that you are interested in, talk to the faculty member who most closely matches your interests. Projects may be available
that are not on this web page, or new projects may be initiated based
on student interest. |
|
|

|
Attenuation of Osteoblast
Apoptosis with Creatinine in
Hibernating Bear Serum
Student Researcher
Sarah Gray, Biomedical Engineering
Advisor
Dr. Seth W. Donahue
Sponsor
Michigan Tech Summer Undergraduate Research
Fellowship (SURF) Michigan Space Grant
Consortium
Project Overview
Currently, forty million Americans are at risk for
developing osteoporosis, and this degenerative
disease accounts for about $20 billion in
health care expenditures annually. Disuse, as in
spaceflight, bed rest, and spinal cord injury, causes
bone to degrade in response to decreased strains.
Bears spend six to eight months out of each year
in hibernation, yet these annual periods of disuse
do not affect their bones. It is hypothesized that
a circulating element in bear serum is attenuating
apoptosis of bone-constructing cells. One serum
element, creatinine, has been strongly correlated
with hibernation, and its apoptosis-inhibiting
qualities have been tested in this study. |
|
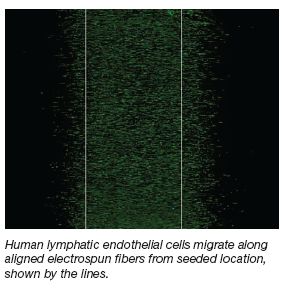 |
Aligned, Electrospun Fibers
Facilitate Treatment of
Lymphedema
Student Researchers
Echoe Bouta and Connor McCarthy, Biomedical
Engineering
Advisors
Dr. Jeremy Goldman and Dr. Ryan Gilbert
Sponsor
National Institutes of Health
Project Overview
When breast cancer patients undergo a
mastectomy, up to 30 percent of patients can
develop lymphedema, a chronic swelling of tissue
in the arm, due to fluid retention, that leads to pain,
disuse of the limb, and poor immune function.
While experimental approaches to promote
lymphangiogenesis utilize growth factors, few
studies utilize topography to direct lymphatic
endothelial cell migration. It is believed that aligned,
electrospun fibers would assist in the directed
migration of lymph endothelial cells. Developing
aligned fiber substrates could help facilitate
regeneration of the injured lymphatic system. |
|
 |
Incorporation of Dextran and
Chitosan Improved Neuron
Attachment and Neurite Extension
Student Researcher
Jonathan Zuidema, Biomedical Engineering
Advisor
Dr. Ryan Gilbert
Sponsors
National Institutes of Health and Michigan Initiative
for Innovation and Entrepreneurship
Project Overview
The material properties of hydrogels are important
for enhancing neuron attachment and neurite
outgrowth. Taking this into account, several
hydrogel blends were created in order to determine
the optimal blend for neuron compatibility. The
surface charge, gel stiffness, gelation time, and
rate of dissolution of the hydrogels were varied and
evaluated. Cortical neurons and dorsal root ganglia
from nine-day-old chicken embryos were seeded
on top of the different blends, and the different
hydrogel blends were then assessed based on
neuron attachment and neurite length. |
|
 |
Self-Diagnosed and Self-Powered
Structures
Student Researcher
Gareth Johnson, Mechanical Engineering
Advisor
Keat Ghee Ong, Biomedical Engineering
Sponsor
SURF
Project Overview
This project focuses on the development of a selfdiagnosed,
self-powered structure based on the
magnetoelastic materials. For the self-diagnosed
part, the magnetoelastic materials were exposed
to a magnetic AC field and their responses at the
harmonic frequencies were captured. Results have
indicated these materials were able to measure
compressive forces, allowing real-time tracking of
surface pressure variations. The same material was
also coupled with piezoelectric materials to convert
magnetic-field induced magnetoelastic vibration
into electrical voltages. The experimental results
have allowed the continuous development of a
smart material that will have important applications
in biomedical and industrial areas. |
|
 |
Light Filtering Polymers for
More Biocompatible Coatings on
Medical Devices
Student Researcher
Genevieve E. Gierke, Biomedical Engineering
Advisor
Dr. Megan C. Frost
Sponsor
SURF
Project Overview
Nitric oxide has been found to reduce the biological
response of implantable devices that are in contact
with blood and tissue. Incorporating light-controlled
nitric oxide-releasing chemicals into polymer
coatings can help to decrease the body’s immune
response. Levels of nitric oxide release, which can
vary according to the wavelengths of light used,
can also cater this response to specific device
needs. The ability to control biological response
would help to decrease the chance of device failure
and undesired complications to patients, making
devices more successful and versatile. |
|
 |
Novel Nitric Oxide Donating
Polymeric Material for Biocompatibility
of Implanted Devices
Student Researcher
Elizabeth Moore, Biomedical Engineering
Advisor
Dr. Megan C. Frost
Sponsor
National Science Foundation
Project Overview
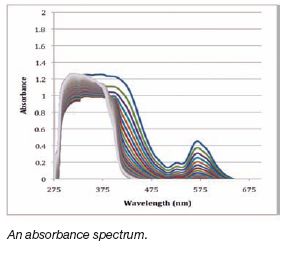
Previous studies have shown that many
S-Nitrosothiols (RSNOs) rapidly degrade, with halflives
from minutes to seconds in aqueous solution.
The research in this paper presents data that the
RSNO 1,3 benzenedinitrosothiol has been relatively
stable for over one year. This RSNO still releases
nitric oxide when subjected to ultraviolet light and
has the same characteristic absorbance peak as a
freshly made RSNO. Developing this stable RSNO
potentially provides a venue for further investigation
into using this nitric oxide donor to improve the
biocompatibility of implanted optical sensors. |
|
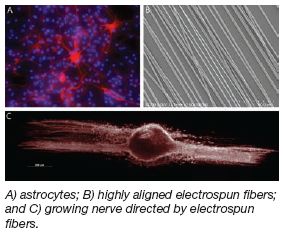 |
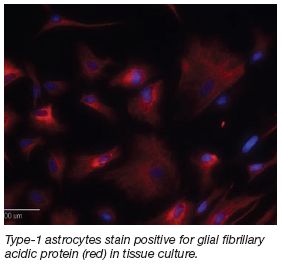
6-Aminonicotinamide Releasing
Highly Aligned PLLA Electrospun
Fibers For Astrocyte Inhibition
Student Researcher
Nick Schaub, Biomedical Engineering
Advisor
Dr. Ryan Gilbert
Sponsor
SURF
Project Overview
Recent inquiry into nerve regeneration using
polymeric fiber scaffolding to direct neurite growth
has shown promising results. The same technique
used to create these polymer nanofibers has
also been a point of interest as a drug-delivery
mechanism, since drug may be released from these
biodegradable fibers. This project combines these
efforts to create aligned nanofibers that release the
drug 6AN in order to metabolically arrest astrocyte
proliferation following spinal cord injury. |
|
 |
Aligned Electrospun Fibers Foster
Axonal Regeneration
Student Researcher
Jared Cregg, Biomedical Engineering
Advisor
Dr. Ryan Gilbert
Sponsor
National Institutes of Health
Project Overview
At present, nearly one in fifty people live with
paralysis, sustaining average yearly health care
costs between $228,566 and $775,567 per
person. Currently there is no clinical paradigm for
treatment; therefore, emphasis has been placed
on understanding injury pathology and developing
combination therapeutic strategies. We investigated
aligned microfiber matrices as a novel platform for
axonal regeneration after a complete transection
spinal cord injury in rats. Aligned microfiber
matrices fostered robust regeneration of axons into
conduit lumen, and, in several animals, permitted
serotonergic axons to navigate the lesion over
twenty-eight days. |
|
 |
Neurite Response Using Highly
Aligned Poly-L-lactic Acid Fibers
as Tissue Scaffolding
Student Researcher
Ryan Young, Chemical Engineering
Advisors
Dr. Ryan Gilbert, Biomedical Engineering, and Dr.
Michael Mullins, Chemical Engineering
Sponsor
SURF
Project Overview
Many approaches to studying human spinal cord
injuries are currently used in laboratory research.
However, virtually every potential treatment is first
studied in vitro. This study’s objective is to create
a standard in vitro model for studying how injured
axons regenerate on aligned topographies. Dorsal
root ganglia (DRG) were placed onto aligned fibers,
and the neurites from the DRG were allowed to
extend for several days. After sufficient neurite
extension, regenerating axons were cut, and the
regeneration behavior examined. By examining
neurite regeneration patterns following transection,
it may be possible to better determine when to
apply therapeutics for injured neurons. |
|
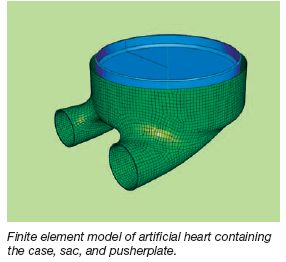 |
Parametric Study of an Artificial
Heart Using Finite Element
Analysis
Student Researcher
Daniel Dubiel, Biomedical Engineering
Advisor
Dr. Tammy Haut Donahue, Mechanical Engineering
Sponsor
Penn State University Hershey Medical School,
Artificial Organs Group
Project Overview
The goal: create a 3-D finite element model to
predict concentrated stresses in a complete
artificial heart. Components used within the model
include a pusher plate, blood sac, and case. A
parametric study was conducted for the blood
sac where stresses hinder longevity of the current
artificial heart. Pressure loads created during the
normal systolic ejection were utilized, along with
constant refinement of element mesh convergence
between components to further the optimization
of the artificial heart model. The final outcome of
the work will dictate the geometry that minimizes
the stresses in the blood sac of the artificial heart,
furthering implant longevity. |
|
|
|

